Grace House Residential Care Home in Farnham uses Docobo Remote Monitoring to enhance resident care and support staff
Read More

Solutions

Our Graphnet Remote Patient Monitoring (RPM) solution powered by Luscii supports patients to take an active role in their health, supporting self-care, improving well-being and transforming out of hospital care.
Combining the Graphnet Remote Monitoring solution with Graphnet’s Population Health management software means that care professionals can identify individuals who will benefit the most from remote patient monitoring, easing the burden on the wider healthcare systems.
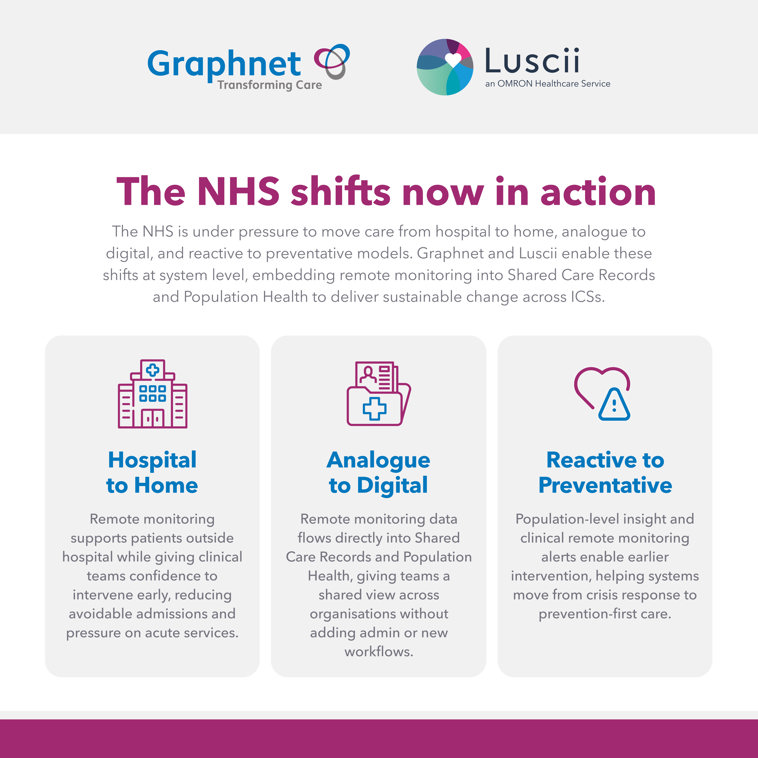
Remote patient monitoring – or RPM – means healthcare providers can deliver care outside of clinical settings, enhancing patients' quality of life at home, in care homes, or other preferred locations.
Using RPM not only benefits the patient but can also help reduce the strain on NHS hospital admissions and lengths of stay.
The NHS’s 10 Year Health Plan forecasts that an additional 500,000 patients will join remote monitoring programmes in the next two years. Graphnet’s partnership with Luscii places us in a unique position to support this transformation by enabling neighbourhood teams to deliver more care in the community and closer to home.
Together, we not only provide trusted tools for remote treatment and monitoring but also deliver powerful population health insights that shift the focus from sickness to prevention. By bringing together digital innovation with real-world clinical pathways, we help the NHS optimise capacity, reduce pressure on hospitals, and empower patients to take a more active role in their own health. This combined approach ensures the shift from analogue to digital is not just about technology, but about creating measurable improvements in outcomes, experience, and long-term financial sustainability.
Graphnet remote monitoring support providers to monitor patients with long-term conditions across a wide range of health services including:
• Heart failure
• Asthma
• COPD
• Atrial fibrillation
• Gestational hypertension and diabetes
• Stroke rehabilitation
• Post transplant surgery
• Macular degeneration
• Peri-operative pain
• Diabetes
• Motor neurone disease
• Frailty
• Cancer
• Paediatrics
• Learning disabilities
• Elderly care
• Post-treatment monitoring
Contact us today to discover how Graphnet Health tools can support your team.
Immunisation Management System (IMS)

Read More

ITV News report on the Docobo Virtual Ward solution from Graphnet
Read More

Read More
© 2026 Graphnet Health Limited . All rights reserved.

